LOW GAMMA AND MATERIALS SCIENCE DATA
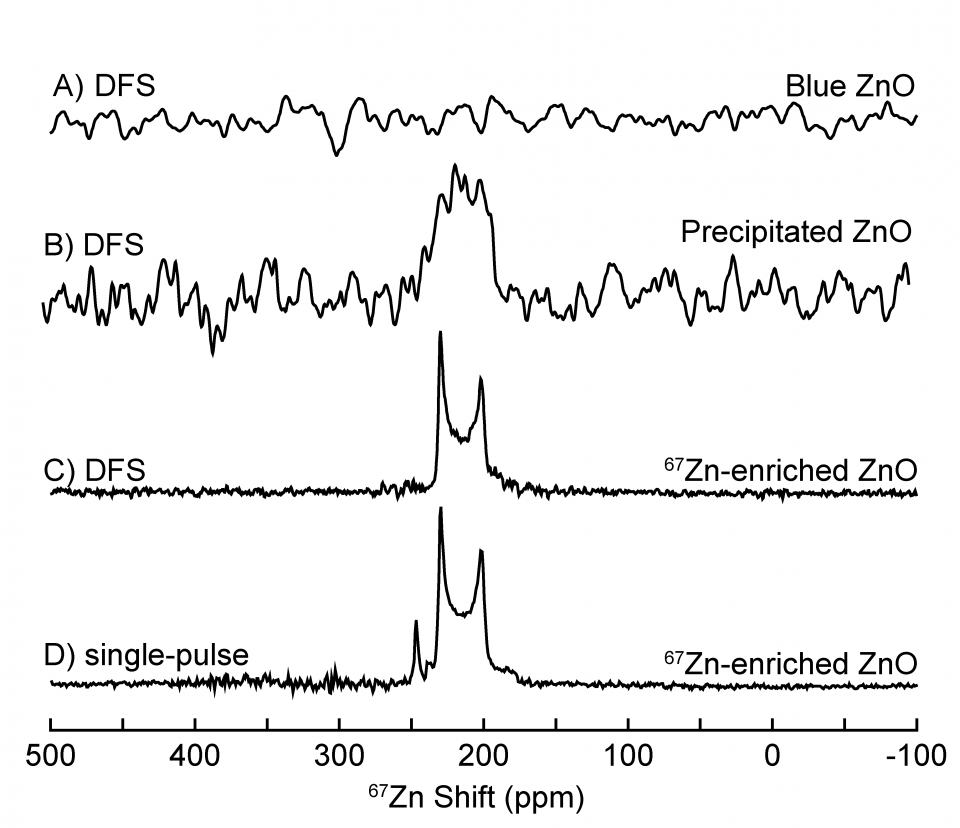
Solid-state 67Zn DFS NMR Data Acquired at 37.5 MHz (14.1 T) using a Phoenix Probe Fitted with a Low-Gamma Accessory
Solid-state 67Zn NMR measurements were acquired of several zinc oxide (ZnO) samples by Robert Messinger and co-workers (1) at 30 kHz MASS using a PhoenixNMR 1.6-mm HXY Premium Probe fitted with a low-gamma accessory and a Bruker Avance III HD 600 MHz NMR spectrometer (67Zn frequency of 37.54 MHz). Single-pulse experiments with π/12 pulses were performed with a double frequency sweep (DFS) prior to acquisition to enhance signal sensitivity(2). A converging DFS was applied for 533 ms, beginning 400 kHz from the central transition and ending 30 kHz from the center of gravity of the central transition to avoid exciting the central transition with the DFS pulse. The DFS improved signal-to-noise of the 67Zn-enriched ZnO by a factor of 1.8 and was used to study Zn-alkaline battery samples. Relaxation delays of 3 seconds were used for all experiments.
Zinc oxide (ZnO) samples were either extracted from zinc metal (Zn) electrodes in aqueous Zn-alkaline batteries or purchased (67Zn-enriched compound). ZnO accumulates in Zn electrodes and leads to increased electrode resistance and battery failure. The data displayed shows three types of ZnO: “Blue ZnO” which was collected from the surface of a Zn electrode at 67Zn natural abundance, “Precipitated ZnO”, which was precipitated from a supersaturated alkaline solution at 67Zn natural abundance, and “67Zn-enriched ZnO”, which was 89.6% 67Zn enriched.
The solid-state 67Zn single-pulse MAS spectrum of the 67Zn-enriched crystalline ZnO, Figure 1D, reveals a central transition lineshape and a satellite transition which disappears upon application of the signal-enhancing DFS, Figure 1C. “Precipitated ZnO” shows a 67Zn signal at a similar shift as the 67Zn-enriched ZnO, Figure 1B, indicating that it exhibits an average 67Zn environment consistent with ZnO. However, a “Blue ZnO” sample retrieved from the electrode surface, which undergoes electrochromic color changes upon cycling, does not yield any solid-state 67Zn NMR signal, Figure 1A, likely due to fast relaxation associated with paramagnetic effects. XRD (not shown) indicates the “Blue ZnO” is indeed wurtzite ZnO, thus establishing that ZnO generated on the surface of Zn electrodes exhibits a significantly different electronic environment compared to ZnO that is precipitated from supersaturated alkaline solution. Understanding the properties of ZnO generated during the electrochemical discharge of Zn metal electrodes will aid in the development of Zn-alkaline batteries.
(1) Data courtesy Prof. Robert Messinger, Brendan E. Hawkins, and Ankur L. Jadhav, Dept. of Chemical engineering, City College of New York, CUNY and the CUNY Energy Institute.
(2) E. van Veenendaal, B.H. Meier, A.P.M. Kentgens, Mol. Phy. 93 (1998) 195.
Figure 1: Solid-state 67Zn NMR spectra taken from single-pulse and DFS measurements of different forms of ZnO. A) DFS spectrum of “Blue ZnO”, B) DFS spectrum of “Precipitated ZnO”, C) DFS spectrum of “67Zn-enriched ZnO” with measured enhancement of 1.8 relative to single-pulse only spectrum, D) single-pulse only (no DFS) spectrum of “67Zn-enriched ZnO”. All spectra were acquired at MASS = 30 kHz.
Solid-state 27Al MQ-MAS NMR Data at 14.1T using PhoenixNMR 1.6 mm HXY probe
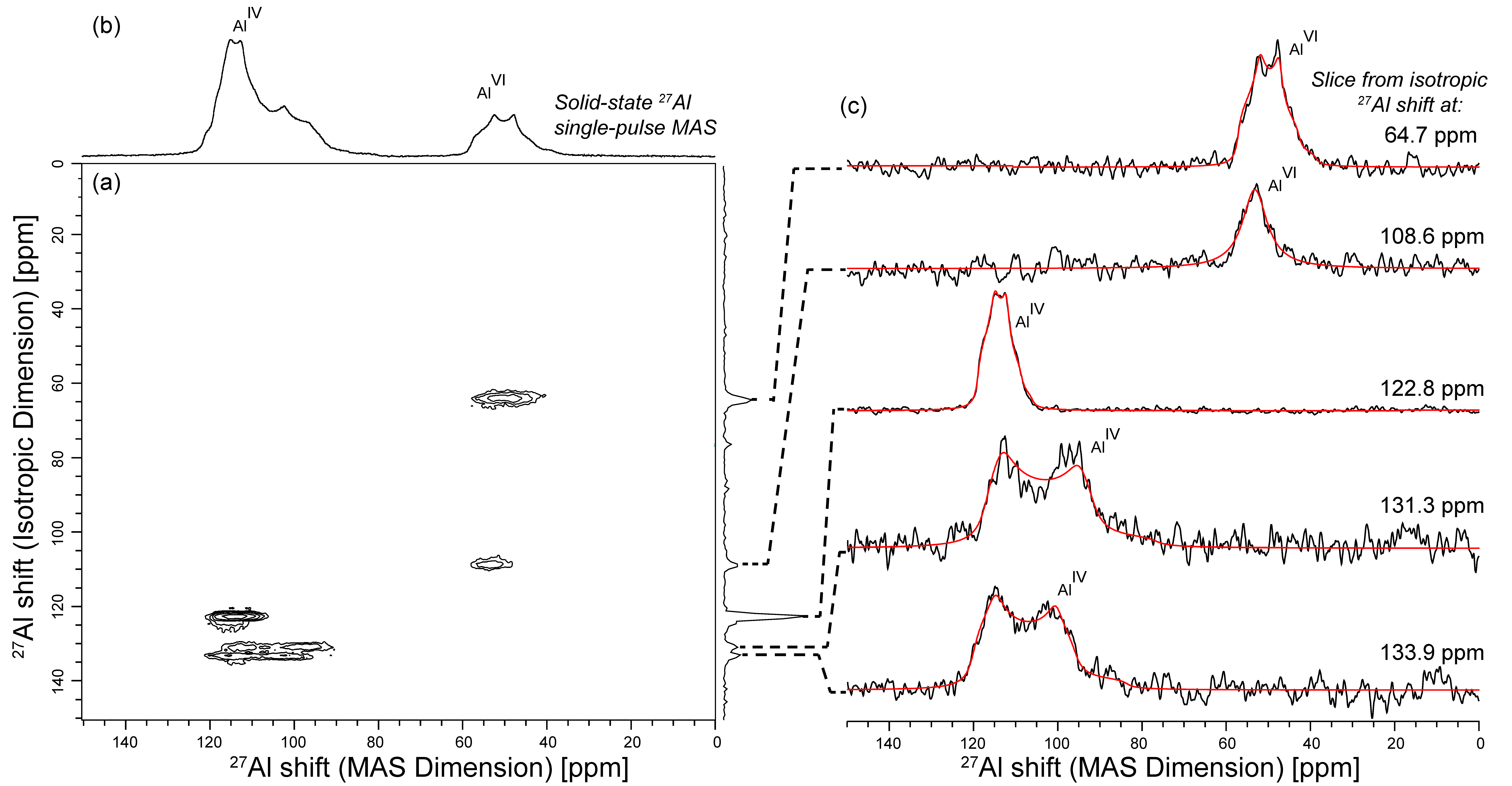
A 2D 27Al MQ-MAS spectrum was acquired for the first time on crystalline Al2S3 (Figure 1a). These 27Al MQ-MAS and single-pulse MAS spectra were acquired by Robert Messinger and co-workers (1) at 30 kHz MASS using a PhoenixNMR 1.6-mm HXY Premium Probe and a Bruker Avance III HD 600 MHz NMR spectrometer (27Al frequency of 156.39 MHz).
Crystalline Al2S3 (98%) was purchased from Sigma-Aldrich. Al2S3 particles were crushed in a mortar and pestle and packed in a 1.6-mm rotor inside an argon-filled glovebox (O2 and H2O levels <1 ppm).
No special spacers or rotor configuration were required to maintain the integrity of this air-sensitive sample during the NMR experiment. Spinning and purge gases were dry air.
The solid-state 27Al single-pulse MAS experiment was performed using a π/12 pulse (0.32 µs) to ensure quantitative linear excitation of all 27Al moieties, regardless of the quadrupolar coupling constant. The solid-state 27Al multiple-quantum (MQ)-MAS NMR spectrum was acquired by correlating triple-quantum (3Q) and central-transition (CT) single-quantum (SQ) coherences using a 3-pulse sequence(2) with excitation, conversion, and CT selective pulses of 3 µs, 1 µs and 18 µs, respectively, and a z-filter delay of 25 µs. The 2D spectrum was subjected to an isotropic shearing transformation. A total of 528 scans were performed along each of the 214 t1-increments in the indirect dimension. Recycle delays of 0.5 s were used for all solid-state 27Al NMR experiments.
As shown in Figure 1b, the single-pulse 27Al MAS spectrum shows overlapping 27Al signals with half-integer quadrupolar lineshapes, which are resolved into five non-equivalent aluminum sites in the 2D MQ-MAS spectrum. 1D projections along the MAS dimension, which contain both isotropic and residual anisotropic interactions, are shown in Figure 1c. Fits to quadrupolar lineshapes elucidate the NMR parameters of each distinct aluminum site in crystalline Al2S3, revealing information about its local electronic environment.
(1) Data courtesy Prof. Robert Messinger, Ankur L. Jadhav, and Rahul Jay, Dept. of Chemical Engineering, City College of New York, CUNY and the CUNY Energy Institute.
(2) J.P. Amoureux, C. Fernandez, S. Steuernagel, J. Magn. Reson. A 123 (1996) 116.
(3) D.Massiot, F.Fayon, M.Capron, I.King, S.Le Calvé, B.Alonso, J.O.Durand, B.Bujoli, Z.Gan, G.Hoatson, Magn. Reson. Chem. 40 (2002) 70.
Figure 1: a) Solid-state 2D 27Al MQ-MAS NMR spectrum of crystalline Al2S3. (b) Separately acquired 1D solid-state 27Al single-pulse MAS spectrum is displayed along the horizontal axis, acquired under quantitative conditions, where 27Al signals associated with four- and six-coordinated aluminum moieties are labeled. (c) 1D slices along the MAS dimension of each of the five non-equivalent aluminum sites resolved in the 2D MQ-MAS spectrum. The slices (black) were fit to quadrupolar lineshapes using the DMFit (3) program (red).